*equal contribution, #co-corresponding authors
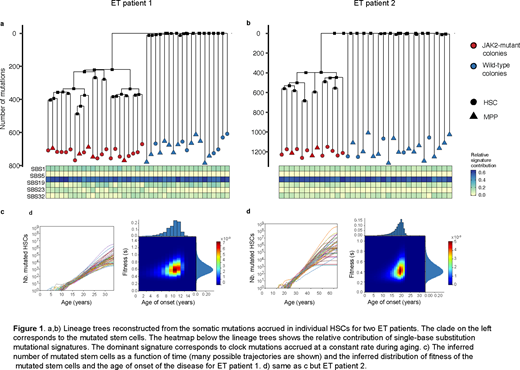
Although the JAK2V617F mutation is the most common MPN phenotypic driver mutation, the precise consequences of the mutation on the behavior of individual human hematopoietic stem cells (HSCs) in vivo remains unknown. We used whole genome sequencing and single-cell profiling of hematopoietic stem and progenitor cells (HSPCs) to quantify the impact of JAK2V617F on the proliferation dynamics of HSCs and the differentiation trajectories of their progenies in individual newly diagnosed MPN patients. We reconstructed the lineage history of individual HSCs obtained from patients with newly diagnosed essential thrombocythemia (ET), using the pattern of spontaneous somatic mutations accrued in their genomes over decades (Figure 1). Intriguingly, our analysis indicates that the JAK2V617F mutation occurred in a single HSC many years before MPN diagnosis - at age 9±2 years in a 34 year-old patient, and at age 19±3 years in a 63 year-old patient. In each patient, we inferred the number of mutated HSCs over the years and computed their fitness. After escaping stochastic extinction, the population of mutated HSCs grew exponentially by 63±15% and 44±13% every year in the two patients respectively.
To contrast the differentiation trajectories of the JAK2-mutant HSCs with those of healthy HSCs, we simultaneously measured the full transcriptome and somatic mutations in single HSPCs in the two ET patients in whom we had performed whole genome sequencing and in one additional ET patient (N=3 total) and also in patients with polycythemia vera (PV) (N=3). We observed, at the time of MPN diagnosis, a consistent lineage bias of JAK2-mutant HSPCs toward megakaryocyte-erythrocyte fate, across ET and PV patients. Exploiting our ability to discriminate JAK2-mutant cells from JAK2 wild-type cells within individual MPN patients, we identified genes involved in antigen presentation and inflammation as differentially up-regulated in JAK2-mutant HSPCs, in particular within the JAK2-mutant CD14+ monocytic cell population. Although we found a range of peripheral blood JAK2V617F variant allele fractions (VAFs) in newly diagnosed ET and PV patients, approximately 20% HSCs in these patients were JAK2-mutant suggesting that peripheral blood mutational burden does not accurately capture the composition and output of JAK2-mutant HSCs.
There are several implications of these findings: first, our studies suggest that the JAK2V617F mutation alone is sufficient to initiate and engender MPN, which has important therapeutic implications. Second, our findings indicate that the JAK2-mutant clone may manifest as clonal hematopoiesis of indeterminate potential (CHIP) for a decade or more before presenting as an overt MPN. This is important not only because JAK2-mutant CHIP has clinical implications, but also because the long "pre-MPN phase" uncovered by our work suggests that MPN could potentially be prevented, by targeting the JAK2-mutant MPN disease-initiating clone prior to exponential expansion. Third, in addition to revealing the limitations of peripheral blood JAK2V617F allele burden measurements, we found that the fraction of JAK2-mutant cells varied significantly across different populations of progenitor cells within the same MPN patient (for example the erythroid progenitors versus the granulocyte-monocyte progenitors). Surprisingly, almost all of the red blood cells, even in ET patients, descended from JAK2-mutant HSCs. Given that the JAK2V617F mutation has been shown to have cell-intrinsic consequences not only in leukocytes, but also in erythroid cells and in platelets, this finding may help explain the development of thrombosis in patients with low peripheral blood JAK2V617F allele burdens.
Together, our study provides the most detailed portrait to date of the functional and transcriptional consequences of the JAK2V617F mutation in individual human HSCs in vivo, and a comprehensive molecular history of MPN initiation and pathogenesis in individual patients. The technology platforms and computational frameworks developed here are broadly applicable to other types of myeloid malignancies and blood cancers.
DeAngelo:Jazz: Consultancy; Incyte Corporation: Consultancy; Forty-Seven: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Agios: Consultancy; Autolos: Consultancy; Amgen: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Glycomimetics: Research Funding; Abbvie: Research Funding; Takeda: Consultancy; Shire: Consultancy. Stone:Syndax: Consultancy, Research Funding; Macrogenics: Consultancy; Syntrix: Other: DSMB; Takeda: Other: DSMB; Stemline: Consultancy; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Arog: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Actinium: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gemoab: Consultancy; Syros: Consultancy; Jazz: Consultancy; Astellas: Consultancy; Aztra-Zeneca: Consultancy; Daiichi-Sankyo: Consultancy; Janssen: Consultancy; Biolinerx: Consultancy; Celgene: Consultancy, Other; Abbvie: Consultancy, Research Funding; Trovagene: Consultancy; Argenix: Other. Garcia:AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; Pfizer: Research Funding; Eli Lily: Research Funding. Hobbs:Jazz: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; Constellation: Honoraria, Research Funding; Bayer: Research Funding; Incyte: Research Funding; Merck: Research Funding. Mullally:Janssen: Consultancy, Research Funding; Actuate Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal